During an elimination reaction, a bond forms by the removal of two atoms or groups from the original molecule. In most instances, the bond that forms is a p bond. Elimination reactions compete with substitution reactions when alkyl halides react with a nucleophile.

The elimination of hydrogen halide (a halogen acid) from an alkyl halide requires a strong base such as the alkoxide ion, RO-. Weaker bases such as the OH- ion give poor yields of elimination product.
If an alkyl halide contains more than two carbons in its chain, and the carbon atoms adjacent to the carbon atom bonded to the halogen each have hydrogen atoms bonded to them, two products will form. The major product is predicted by Zaitsev's Rule, which states that the more highly branched alkene will be the major product. For example, in the dehydrohalogenation reaction between 2-chlorobutane and sodium methoxide, the major product is 2-butene.

Mechanism of Elimination Reactions
As noted earlier, the halogen-carbon bond in an alkyl halide is polarized due to the electronegativity difference between the atoms. This polarization can lead to the formation of a partial or fully positive charge on the carbon atom

The full or partial positive charge on the carbon atom is delocalized (dispersed) down the carbon chain. This, in turn, makes the hydrogen atoms attached to these carbons very slightly positive and thus very weakly acidic. Therefore, a very strong base can now remove slightly positive hydrogen with the resulting release of electrons down the chain, forming a p bond between the carbon atoms. The actual mechanism can be one of two types, E1 or E2, depending upon the structure of the activated complex.
E1 mechanism
An atom that bears a pair of unshared electrons takes on one of two roles. The atom may share these electrons with a carbon atom that bears a leaving group, or it may share these electrons with a hydrogen atom. In the former case, the atom acts as a nucleophile, while in the latter case it acts as a base. Therefore, depending on reaction conditions, the atom may be involved in a substitution reaction or an elimination reaction.
The reaction of an OH- ion with tertiary butyl bromide leads to little or no substitution product because steric hindrance blocks the rear lobe of the carbon atom to which the bromine atom is bonded. With the aid of a polar solvent, the bromine-carbon bond ionizes to form a tertiary carbocation and a bromide ion. The hydrogen atoms on the carbons adjacent to the carbocation carbon acquire a slight positive charge, allowing the OH- ion to employ its basic characteristics. Thus, the OH- ion abstracts a hydrogen atom, and the electrons migrate down the chain, forming a double bond.

The activated complex for this reaction contains only the alkyl halide and is, therefore, unimolecular. The reaction follows an E1 mechanism

E2 mechanism
Elimination reactions can also occur when a carbon halogen bond does not completely ionize, but merely becomes polarized. As with the E1 reactions, E2 mechanisms occur when the attacking group displays its basic characteristics rather than its nucleophilic property. The activated complex for this mechanism contains both the alkyl halide and the alkoxide ion.
Following is the complete mechanism for the E2 elimination reaction:

Grignard Reaction
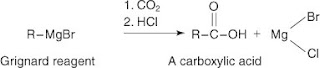
In a Grignard reaction, an alkyl halide reacts with magnesium metal in an anhydrous ether solvent to create an organometallic reagent

The Grignard reagent is highly reactive and is used to prepare many functional groups. An example is the preparation of a carboxylic acid by reaction with carbon dioxide and mineral acid.