Nucleophilic Substitution Reactions
Alkyl halides undergo many reactions in which a nucleophile displaces the halogen atom bonded to the central carbon of the molecule. The displaced halogen atom becomes a halide ion.

Some typical nucleophiles are the hydroxy group (-OH), the alkoxy group (RO-), and the cyanide ion (-C N). Reaction of these nucleophiles with an alkyl halide (R—X) gives the following reactions and products:

The halogen ion that is displaced from the carbon atom is called the leaving group, and the overall reaction is called a nucleophilic substitution reaction.
Leaving Group
For a molecule to act as a nucleus or substrate in a nucleophilic substitution reaction, it must have both a polar bond and a good leaving group. For an atom or a group to be a good leaving group, it must be able to exist independently as a relatively stable, weakly basic ion or molecule. Groups that act as leaving groups are always capable of accommodating the negative charge through a high electronegativity or by delocalization. Because halogen atoms have high electronegativities and form relatively stable ions, they act as good leaving groups.
Nucleophilic Substitution Reactions: Mechanisms
Experimental data from nucleophilic substitution reactions on substrates that have optical activity (the ability to rotate plane-polarized light) shows that two general mechanisms exist for these types of reactions. The first type is called an SN2 mechanism. This mechanism follows second-order kinetics (the reaction rate depends on the concentrations of two reactants), and its intermediate contains both the substrate and the nucleophile and is therefore bimolecular. The terminology SN2 stands for “substitution nucleophilic bimolecular.”
The second type of mechanism is an SN1 mechanism. This mechanism follows first-order kinetics (the reaction rate depends on the concentration of one reactant), and its intermediate contains only the substrate molecule and is therefore unimolecular. The terminology SN1 stands for “substitution nucleophilic unimolecular.”
SN2 mechanism
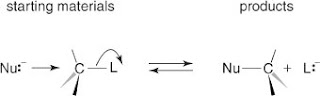
The alkyl halide substrate contains a polarized carbon halogen bond. The SN2 mechanism begins when an electron pair of the nucleophile attacks the back lobe of the leaving group. Carbon in the resulting complex is trigonal bipyramidal in shape. With the loss of the leaving group, the carbon atom again assumes a pyramidal shape; however, its configuration is inverted. See Figure below.

The SN2 mechanism can also be illustrated as shown in Figure .

Notice that in either picture, the intermediate shows both the nucleophile and the substrate. Also notice that the nucleophile must always attack from the side opposite the side that contains the leaving group. This occurs because the nucleophilic attack is always on the back lobe (antibonding orbital) of the carbon atom acting as the nucleus.
SN2 mechanisms always proceed via rearward attack of the nucleophile on the substrate. This process results in the inversion of the relative configuration, going from starting material to product. This inversion is often called the Walden inversion, and this mechanism is sometimes illustrated as shown in Figure
Steric hindrance
SN2 reactions require a rearward attack on the carbon bonded to the leaving group. If a large number of groups are bonded to the same carbon that bears the leaving group, the nucleophile's attack should be hindered and the rate of the reaction slowed. This phenomenon is called steric hindrance. The larger and bulkier the group(s), the greater the steric hindrance and the slower the rate of reaction. Table shows the effect of steric hindrance on the rate of reaction for a specific, unspecified nucleophile and leaving group. Different nucleophiles and leaving groups would result in different numbers but similar patterns of results.
TABLE 1 Effects of Steric Hindrance upon Rates of SN2 Reactions
Alkyl Group (ALK) Relative Rate of Substitution
-CH3(small group) 30
-CH2CH3 (larger group) 1
-CH(CH3)2 (bulky group) 0.03
-C(CH3)3 (very bulky group) 0
SN2 reactions give good yields on 1° (primary) alkyl halides, moderate yields on 2° (secondary) alkyl halides, and poor to no yields on 3° (tertiary) alkyl halides.
Solvent effects
For protic solvents (solvents capable of forming hydrogen bonds in solution), an increase in the solvent's polarity results in a decrease in the rate of SN2 reactions. This decrease occurs because protic solvents solvate the nucleophile, thus lowering its ground state energy. Because the energy of the activated complex is a fixed value, the energy of activation becomes greater and, therefore, the rate of reaction decreases.
Polar aprotic solvents (solvents that cannot form hydrogen bonds in solution) do not solvate the nucleophile but rather surround the accompanying cation, thereby raising the ground state energy of the nucleophile. Because the energy of the activated complex is a fixed value, the energy of activation becomes less and, therefore, the rate of reaction increases.
Figure illustrates the effect of solvent polarity on the energy of activation and, thus, the rate of reaction.

The smaller activation energy leads to the more rapid reaction.
