
Wednesday 27 May 2009
Preparation of Alkyl Halides
Preparation of Alkyl Halides
Following are two methods commonly used to prepare alkyl halides.
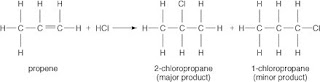
Hydrogen halide addition to an alkene
Halogen halides add across carbon-carbon double bonds. These additions follow Markovnikov's rule, which states that the positive part of a reagent (a hydrogen atom, for example) adds to the carbon of the double bond that already has more hydrogen atoms attached to it. The negative part adds to the other carbon of the double bond. Such an arrangement leads to the formation of the more stable carbocation over other less-stable intermediates.
Reaction of alcohols with sulfur and phosphorous halides
Alcohols can be converted to alkyl halides by reaction with thionyl chloride, SOCl2•, phosphorous trichloride, PCl3•, phosphorous pentachloride, PCl5•, or phosphorous tribromide, PBr3. For example, ethyl chloride or ethyl bromide can be prepared from ethyl alcohol via reactions with sulfur and phosphorous halides.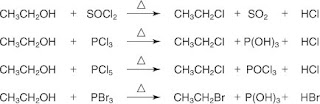
Elimination Reactions
During an elimination reaction, a bond forms by the removal of two atoms or groups from the original molecule. In most instances, the bond that forms is a p bond. Elimination reactions compete with substitution reactions when alkyl halides react with a nucleophile.

The elimination of hydrogen halide (a halogen acid) from an alkyl halide requires a strong base such as the alkoxide ion, RO-. Weaker bases such as the OH- ion give poor yields of elimination product.
If an alkyl halide contains more than two carbons in its chain, and the carbon atoms adjacent to the carbon atom bonded to the halogen each have hydrogen atoms bonded to them, two products will form. The major product is predicted by Zaitsev's Rule, which states that the more highly branched alkene will be the major product. For example, in the dehydrohalogenation reaction between 2-chlorobutane and sodium methoxide, the major product is 2-butene.

Mechanism of Elimination Reactions
As noted earlier, the halogen-carbon bond in an alkyl halide is polarized due to the electronegativity difference between the atoms. This polarization can lead to the formation of a partial or fully positive charge on the carbon atom

The full or partial positive charge on the carbon atom is delocalized (dispersed) down the carbon chain. This, in turn, makes the hydrogen atoms attached to these carbons very slightly positive and thus very weakly acidic. Therefore, a very strong base can now remove slightly positive hydrogen with the resulting release of electrons down the chain, forming a p bond between the carbon atoms. The actual mechanism can be one of two types, E1 or E2, depending upon the structure of the activated complex.
E1 mechanism
An atom that bears a pair of unshared electrons takes on one of two roles. The atom may share these electrons with a carbon atom that bears a leaving group, or it may share these electrons with a hydrogen atom. In the former case, the atom acts as a nucleophile, while in the latter case it acts as a base. Therefore, depending on reaction conditions, the atom may be involved in a substitution reaction or an elimination reaction.
The reaction of an OH- ion with tertiary butyl bromide leads to little or no substitution product because steric hindrance blocks the rear lobe of the carbon atom to which the bromine atom is bonded. With the aid of a polar solvent, the bromine-carbon bond ionizes to form a tertiary carbocation and a bromide ion. The hydrogen atoms on the carbons adjacent to the carbocation carbon acquire a slight positive charge, allowing the OH- ion to employ its basic characteristics. Thus, the OH- ion abstracts a hydrogen atom, and the electrons migrate down the chain, forming a double bond.

The activated complex for this reaction contains only the alkyl halide and is, therefore, unimolecular. The reaction follows an E1 mechanism

E2 mechanism
Elimination reactions can also occur when a carbon halogen bond does not completely ionize, but merely becomes polarized. As with the E1 reactions, E2 mechanisms occur when the attacking group displays its basic characteristics rather than its nucleophilic property. The activated complex for this mechanism contains both the alkyl halide and the alkoxide ion.
Following is the complete mechanism for the E2 elimination reaction:

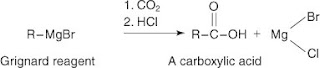
Grignard Reaction
In a Grignard reaction, an alkyl halide reacts with magnesium metal in an anhydrous ether solvent to create an organometallic reagent

The Grignard reagent is highly reactive and is used to prepare many functional groups. An example is the preparation of a carboxylic acid by reaction with carbon dioxide and mineral acid.
SN1 mechanism
SN1 mechanism
The second major type of nucleophilic substitution mechanism is the SN1 mechanism. This mechanism proceeds via two steps. The first step (the slow step) involves the breakdown of the alkyl halide into an alkyl carbocation and a leaving group anion. The second step (the fast step) involves the formation of a bond between the nucleophile and the alkyl carbocation.



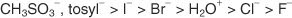
Life
Life
Though there is no universal agreement on the definition of life, scientists generally accept that the biological manifestation of life is characterized by organization, metabolism, growth, adaptation, response to stimuli and reproduction. Life may also be said to be simply the characteristic state of organisms.
Properties common to terrestrial organisms are that they are cellular, carbon-and-water-based with complex organization, having a metabolism, a capacity to grow, respond to stimuli, and reproduce. An entity with these properties is generally considered life. However, not every definition of life considers all of these properties to be essential. Human-made analogs of life may also be considered to be life.
Wilderness is commonly defined as a natural environment on Earth that has not been significantly modified by human activity. The Wild foundation goes into more detail, defining wilderness as: "The most intact, undisturbed wild natural areas left on our planet - those last truly wild places that humans do not control and have not developed with roads, pipelines or other industrial infrastructure." Wilderness areas and protected parks are considered important for the survival of certain species, ecological studies, conservation, and recreation. Wilderness is deeply valued for cultural, spiritual, moral, and aesthetic reasons. Some nature writers believe wilderness areas are vital for the human spirit and creativity. The word, "wilderness", derives from the notion of wildness; in other words that which is not controllable by humans. The word's etymology is from the old English wildeornes , which in turn derives from wildeor meaning wild best (wild + deor = beast, deer). From this point of view, it is the wildness of a place that makes it a wilderness. The activity of people does not disqualify an area from being "wilderness." Many ecosystems that are influenced by activities of people may still be considered "wild." This way of looking at wilderness includes areas within which natural processes operate without very noticeable human interference.
Climate
The temperatures, humidity, atmospheric pressure, winds, rainfall, atmospheric particle count and numerous other meteorological elements in a given region over long periods of time, as opposed to the term weather, which refers to current activity of these same elements known as Climate. The climate of a location is affected by its latitude, terrain, altitude, persistent ice or snow cover, as well as nearby oceans and their currents. Climates have to be classified using parameters such as temperature and rainfall to define specific climate types. The Thornthwaite system, in use since 1948, incorporates evapotranspiration in addition to temperature and precipitation information and is used in studying animal species diversity and potential impacts of climate change.
Paleoclimatology is the study and description of ancient climates using information from both non-biotic factors such as sediments found in lake beds and ice cores, and biotic factors such as tree rings and coral, and can be used to extend back the temperature or rainfall information for particular locations to a time before various weather instruments were used to monitor weather conditions. Climate mode are mathematical models of past, present and future climates and can be used to describe the likely patterns of future changes.
Defination of Climate:
Climate (from Ancient Greek klima, meaning inclination) is defined as the weather averaged over a long period of time. The standard averaging period is 30 years, but other periods may be used depending on the purpose. Climate also includes statistics other than the average, such as the magnitudes of day-to-day or year-to-year variations. The Intergovernmental Panel on Climate Change glossary definition is:“
Climate in a narrow sense is usually defined as the "average weather," or more rigorously, as the statistical description in terms of the mean and variability of relevant quantities over a period of time ranging from months to thousands or millions of years. The classical period is 30 years, as defined by the World Meteorological Organization. These quantities are most often surface variables such as temperature, precipitation, and wind. Climate in a wider sense is the state, including a statistical description, of the climate system
The difference between climate and weather is usefully summarized by the popular phrase "Climate is what you expect, weather is what you get. Over historical time spans there are a number of static variables that determine climate, including latitude, altitude, proportion of land to water, and proximity to oceans and mountains.
Definition of weather
Weather is the sequence of the states of the atmosphere as time passes. We know, the behavior of the atmosphere at a given place can be described with number of quantities characterizing the physical state of the air, such as its temperature, pressure, water content and motion .
Generally, we define climate as an ensemble of all the states of the atmosphere at a place experienced in the course of years and over the years of some large but finite time interval. The expression of the length of the time interval could be ’sufficiently long’ but finite’, for which we have a free choice to take a sample of the atmosphere’s states, does not seem very scientific.
Though weather is the sequence of states of the atmosphere, it is tempting to define a ”true” climate in terms of a limit as the time interval approaches infinity, e.g. it is the total ensemble of the atmospheric states which ever occurred in the past. But the questions arise: how can we arrive at and what can we do with such an abstract idea? The practical difficulty is computing the statistical properties of an infinite time series, what is impossible. The theoretical difficulty is that we would not be able to consider the change of this type of climate.
Universally accepted convention recommended by the World Meteorological Organization that the 30-year period is a basic climatic time scale, and the statistical properties calculated for the consecutive 30-year periods 1901-1930, 1931-1960, and most frequently used 1961-90. Are considered and called climatologically standard normal.
Now at present the climate is changing rapidly, climate characteristics are sometimes re-calculated every 10 years for the period of the recent 30 years, i.e. 1961 - 1990, 1971 - 2000, ... although the next official period would be 1991 - 2020. These fixed time intervals allow world-wide comparison of climatological events. For example, the suitable climatic time scales are completely different in case of initiation of the cultivation of a new plant from those needed for investigating the glacial chronology.
Pollution
Pollution is the process of making air, water, soil, etc. dirty; the state of being dirty. Pollution is also the introduction of contaminants into an environment that causes instability, disorder, harm or discomfort to the ecosystem i.e. physical systems or living organisms. Pollution can take the form of chemical substances such as noise, heat, or light energy. Pollutants, the elements of pollution, can be foreign substances or energies, or naturally occurring; when naturally occurring, they are considered contaminants when they exceed natural levels. Pollution is often classed as point source or nonpoint source pollution.
Air pollution
Air pollution is occurred by the release of chemicals and particulates into the atmosphere. Common gaseous air pollutants include carbon dioxide (CO2 ), sulfur dioxide (SO2), chlorofluorocarbons (CFC) and nitrogen oxides (NO2) produced by industry and motor vehicles. Photochemical ozone and smog are created as nitrogen oxides and hydrocarbons react to sunlight.
Water pollution
Water pollution is occurred by the release of waste products and contaminants into surface runoff into river drainage systems, leaching into groundwater, liquid spills, wastewater discharges and littering.
Soil contamination occurs when chemicals are released by spill or underground leakage. Among the most significant soil contaminants are hydrocarbons, heavy metals, herbicides, pesticides and chlorinated hydrocarbons.
Sources and causes
Air pollution is created by both natural and manmade sources. Though globally manmade pollutants from combustion, construction, mining, agriculture and warfare are increasingly significant in the air pollution equation.
Motor vehicle emissions are one of the leading causes of air pollution. Japan , United States , China , Mexico and Russia
Some of the more common soil contaminants are chlorinated hydrocarbons (CFH), heavy metals (such as chromium, cadmium--found in rechargeable batteries, and lead--found in lead paint, aviation fuel and still in some countries, gasoline), MTBE, zinc, arsenic and benzene. In 2001 a series of press reports culminating in a book called Fateful Harvest unveiled a widespread practice of recycling industrial byproducts into fertilizer, resulting in the contamination of the soil with various metals. Ordinary municipal landfills are the source of many chemical substances entering the soil environment (and often groundwater), emanating from the wide variety of refuse accepted, especially substances illegally discarded there, or from pre-1970 landfills that may have been subject to little control in the U.S. or EU. There have also been some unusual releases of polychlorinated dibenzodioxins, commonly called dioxins for simplicity, such as TCDD.
Pollution can also be the consequence of a natural disaster. For example, hurricanes often involve water contamination from sewage, and petrochemical spills from ruptured boats or automobiles. Larger scale and environmental damage is not uncommon when coastal oil rigs or refineries are involved. Some sources of pollution, such as nuclear power plants or oil tankers, can produce widespread and potentially hazardous releases when accidents occur.
In the case of noise pollution the dominant source class is the motor vehicle, producing about ninety percent of all unwanted noise worldwide.
Environment
Environment
The environment is the symbiosis between the physical environment and the biological life forms within the environment, and includes all variables that comprise the Earth’s biosphere. The environment can be divided into two categories: the natural environment and the built environment, with some overlap between the two.The built environment has become an increasingly significant part of the Earth's environment.
Constituents
The scope of the environment is all that contained in the biosphere, which is that part of the Earth in which all life occurs. Ecosystems of which there are numerous types and are a defined part of the biosphere, collectively make up the whole of the biosphere. Within an ecosystem there are habitats in which an organism exists. At its most natural, an environment would lack any effects of human activity, although the scale of this activity is such that all areas of the Earth have had at least some influence by humans. At the other end of the scale is the built environment. The environment can vary in scale from microscopic to global in extent. They can also be subdivided according to their attributes. Some examples may be the marine environment, the atmospheric environment and the terrestrial environment.
Environmental science
Environmental science is defined as the study of the interactions within the biophysical environment. Part of this discipline is the investigation of the effect of human activity on the environment. Ecology, a sub-discipline of biology and a part of environmental sciences, is often mistaken as a study of human induced effects on the environment. Environmental science is a broader academic discipline that is the systematic study of interaction of humans with their environment. It is a broad field of study that includes the natural environment, built environments and social environments.
Natural environment
The natural environment is generally referred to as the environment, is a term that encompasses all living and non-living things occurring naturally on Earth. The concept of the natural environment can be distinguished by components:
Complete ecological units that function as natural systems without massive human intervention, including all vegetation, animals, microorganism, soil, rocks, atmosphere and natural phenomena that occur within their boundaries.
Universal natural resources and physical phenomena that lack clear-cut boundaries, such as air , water and climate, as well as energy radiation electric charge and magnetism, not originating from human activity.
The natural environment is contrasted with the built environment, which comprises the areas and components that are strongly influenced by humans. A geographical area is regarded as a natural environment, if the human impact on it is kept under a certain limited level.
Built environment
The built environment is the man-made surroundings that provide the setting for human activity, ranging from the large-scale civic surroundings to the personal places. The term is also now widely used to describe the interdisciplinary field of study which addresses the design and use of these man-made surroundings and their relationship to the human activities which take place within them. The field is generally not regarded as an academic discipline in its own right, but as a "field of application" which draws upon the individual disciplines of economics, law, management, design and technology in sustainable sense. In architecture and environmental psychology, the phrase is a useful acknowledgement that a small fraction of buildings constructed annually, even in the industrialized world, are designed by architects, and that users of the built environment encounter issues that cross the traditional professional boundaries between traffic engineers, zoning authorities, architects, interior designer, industrial designers etc. Historically, much of the built environment has taken the form of vernacular, and this is still the case in large parts of the world. In the industrialized world, many buildings are produced by large scale development remote from its eventual users. In landscape architecture, the built environment is identified as opposed to the natural environment, with the recognition that places like Central Park may have the look, feel, and nourishing quality of natural surroundings while being completely artificial and "built," thus blurring the line between the two. Recently there has also been considerable dialogue and research into the impact of the built environment's impact on population health.
Environmental Chemistry
Definition of environmental chemistry :
Environmental chemistry is the study of the chemical and biochemical phenomena that happen in natural places. It should not be confused with green chemistry, which seeks to reduce potential pollution at its source. It is defined as the study of the sources, reactions, transport, effects, and fates of chemical species in the air, soil and water environments; and the effect of human activity on these. Environmental chemistry is an interdisciplinary science that includes atmospheric, aquatic, and soil chemistry, as well as heavily relying on analytical chemistry and being related to environmental and other areas of science.
chemistry is that branch of one, which deals with the study of chemical and biochemical phenomena that occur in natural places like air, soil, water.
Environmental chemistry involves first understanding how the uncontaminated environment works, which chemicals in what concentrations are present naturally, and with what effects. Without this it would be impossible to accurately study the effects humans have on the environment through the release of chemicals .
Environmental chemists draw on a range of concepts from chemistry and various environmental sciences to assist in their study of what is happening to a chemical species in the environment.
Nucleophilic Substitution Reactions
Nucleophilic Substitution Reactions
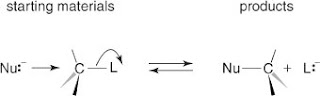
Alkyl halides undergo many reactions in which a nucleophile displaces the halogen atom bonded to the central carbon of the molecule. The displaced halogen atom becomes a halide ion.

Some typical nucleophiles are the hydroxy group (-OH), the alkoxy group (RO-), and the cyanide ion (-C N). Reaction of these nucleophiles with an alkyl halide (R—X) gives the following reactions and products:




Introduction to Alkyl Halides
An alkyl halide is another name for a halogen-substituted alkane. The carbon atom, which is bonded to the halogen atom, has sp3 hybridized bonding orbitals and exhibits a
tetrahedral shape. Due to electronegativity differences between the carbon and halogen atoms, the s covalent bond between these atoms is polarized, with the carbon atom becoming slightly positive and the halogen atom partially negative. Halogen atoms increase in size and decrease in electronegativity going down the family in the periodic table. Therefore, the bond length between carbon and halogen becomes longer and less polar as the halogen atom changes from fluorine to iodine.
Physical properties
Alkyl halides have little solubility in water but good solubility with nonpolar solvents, such as hexane. Many of the low molecular weight alkyl halides are used as solvents in reactions that involve nonpolar reactants, such as bromine. The boiling points of different alkyl halides containing the same halogen increase with increasing chain length. For a given chain length, the boiling point increases as the halogen is changed from fluorine to iodine. For isomers of the same compound, the compound with the more highly-branched alkyl group normally has the lowest boiling point. Table summarizes data for some
representative alkyl halides.
TABLE 1 Boiling Points (°C) of Alkyl Halides
Flouride Chloride Bromide Iodine
Group bp bp bp bp
Methyl -78.4 -28.8 -3.6 42.5
Ethyl -37.7 13.1 38.4 72
Propyl -2.5 46.6 70.8 102
Isopropyl -9.4 34 59.4 89.4
Butyl 32 78.4 101 130
Sec-butyl 68 91.2 120
Tert-butyl 51 73.3 100
Nomenclature
Alkyl halides are named using the IUPAC rules for alkanes. Naming the alkyl group attached to the halogen and adding the inorganic halide name for the halogen atom creates common names.



