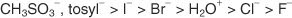SN1 mechanism
The second major type of nucleophilic substitution mechanism is the SN1 mechanism. This mechanism proceeds via two steps. The first step (the slow step) involves the breakdown of the alkyl halide into an alkyl carbocation and a leaving group anion. The second step (the fast step) involves the formation of a bond between the nucleophile and the alkyl carbocation.

Because the activated complex contains only one species—the alkyl carbocation—the substitution is considered unimolecular.
Carbocations contain sp2 hybridized orbitals and thus have planar structures. SN1 mechanisms proceed via a carbocation intermediate, so a nucleophile attack is equally possible from either side of the plane. Therefore, a pure, optically active alkyl halide undergoing an SN1 substitution reaction will generate a racemic mixture as a product, as shown in Figure .

SN1 versus SN2 Reactions
Whether an alkyl halide will undergo an SN1 or an SN2 reaction depends upon a number of factors. Some of the more common factors include the natures of the carbon skeleton, the solvent, the leaving group, and the nature of the nucleophile.
Nature of the carbon skeleton
Only those molecules that form extremely stable cations undergo SN1 mechanisms. Normally, only compounds that yield 3° (tertiary) carbonications (or resonance-stabilized carbocations) undergo SN1 mechanisms rather than SN2 mechanisms. Carbocations of tertiary alkyl halides not only exhibit stability due to the inductive effect, but the original molecules exhibit steric hindrance of the rear lobe of the bonding orbital, which inhibits SN2 mechanisms from occurring. Primary alkyl halides, which have little inductive stability of their cations and exhibit no steric hindrance of the rear lobe of the bonding orbital, generally undergo SN2 mechanisms. Figure illustrates the tendencies of alkyl halides toward the two types of substitution mechanisms.
Nature of the solvent
Polar protic solvents such as water favor SN1 reactions, which produce both a cation and an anion during reaction. These solvents are capable of stabilizing the charges on the ions formed during solvation. Because SN2 reactions occur via a concerted mechanism (a mechanism which takes place in one step, with bonds breaking and forming at the same time) and no ions form, polar protic solvents would have little effect upon them. Solvents with low dielectric constants tend not to stabilize ions and thus favor SN2 reactions. Conversely, solvents of high dielectric constants stabilize ions, favoring SN1 reactions.
Nature of the leaving group
In general, good leaving groups are those capable of forming stable ions or molecules upon displacement from the original molecule. Conversely, poor leaving groups form ions of poor to moderate stability. Strong bases, such as OH-, NH2-, and RO-, make poor leaving groups. Water, which is less basic than a hydroxide ion, is a better leaving group. Poor bases usually make good leaving groups. A poor base is an ion or group in which the electrons are tightly bound to the molecule due to high electronegativity or resonance. Some good leaving groups are the sulfate ion and the p-toluenesulfonate (tosylate ion).

The following list ranks atoms and molecules in order of their stability as leaving groups, from most to least stable