Organic chemistry
Organic chemistry is the scientific study of the structure, properties, composition, reactions, and synthesis of organic compounds. Organic compounds are composed of carbon and hydrogen, and can possibly contain any of the other elements such as nitrogen, oxygen, phosphorus, and sulfur.
History
Organic chemistry as a science is generally agreed to have started in 1828 with Friedrich Woehler's synthesis of the organic, biologically significant compound urea by accidentally evaporating an aqueous solution of ammonium cyanate NH4OCN.
Characteristics of organic substances
The reason that there are so many carbon compounds is that carbon has the ability to form many carbon chains of different lengths, and rings of different sizes (catenation). Many carbon compounds are extremely sensitive to heat, and generally decompose below 300°C. They tend to be less soluble in water compared to many inorganic salts. In contrast to such salts, they tend to be much more soluble in organic solvents such as ether or alcohol. Organic compounds are covalently bonded.
Aliphatic compounds
Aliphatic compounds are organic molecules that do not contain aromatic systems. ....
Hydrocarbons - Alkanes- Alkenes - Diene or Alkadienes - Alkynes - Halogenoalkanes
Aromatic compounds
Aromatic compounds are organic molecules that contain one or more aromatic ring system.
Benzene - Toluene - Styrene- Xylene- Aniline - Phenol - Acetophenone - Benzonitrile - Halogenoarenes -Naphthalene- Anthracene- Phenanthrene- Benzopyrene - Coronene- Azulene - Biphenyl
Heterocyclic compounds
Heterocyclic compounds are cyclic organic molecules whose ring(s) contain at least one heteroatom. These heteroaoms can include oxygen, nitrogen, phosphorous, and sulfur.
Pyridine - Pyrrole- Thiophene - Furan
Functional groups
Alcohols - Mercaptans - Ethers - Aldehydes- Ketones- Carboxylic acids - Esters - Carbohydrates -Alicyclic compounds - Amides- Amines - Lipids - Nitriles
Polymers
Polymers are a special kind of molecule. Generally considered "large" molecules, polymers get their reputation regarding size because they are molecules that consist of multiple smaller segments. The
segments could be chemically identical, which would make such a molecule a homopolymer. Or the segments could be vary in chemical structure, which would make that molecule a heteropolymer. Polymers are a subset of "macromolecules" which is just a classification for all molecules that are considered large.
Polymers can be organic or inorganic. Commonly-encountered polymers are usually organic (e.g.,polyethylene, polypropylene, Plexiglass, etc.). But inorganic polymers (e.g., silicone) are also familiar to everyday items.
Organic nomenclature
Organic nomenclature is the system established for naming and grouping organic compounds.
Formally, rules established by the International Union of Pure and Applied Chemistry (known as IUPAC nomenclature) are authoritative for the names of organic compounds, but in practice, a number of simply-applied rules can allow one to use and understand the names of many organic compounds.
For many compounds, naming can begin by determining the name of the parent hydrocarbon and by identifying any functional groups in the molecule that distinguish it from the parent hydrocarbon. The numbering of the parent alkane is used, as modified, if necessary, by application of the Cahn Ingold Prelog priority rules in the case that ambiguity remains after consideration of the structure of the
parent hydrocarbon alone. The name of the parent hydrocarbon is modified by the application of the highest-priority functional group suffix, with the remaining functional groups indicated by numbered prefixes, appearing in the name in alphabetical order from first to last.
In many cases, lack of rigor in applying all such nomenclature rules still yields a name that is intelligible — the aim, of course, being to avoid any ambiguity in terms of what substance is being discussed.
For instance, strict application of CIP priority to the naming of the compound
NH2CH2CH2OH
would render the name as 2-aminoethanol, which is preferred. However, the name 2-hydroxyethanamine unambiguously refers to the same compound.
How the name was constructed:
- There are two carbons in the main chain; this gives the root name "eth".
- Since the carbons are singly-bonded, the suffix begins with "an".
- The two functional groups are an alcohol (OH) and an amine (NH2). The alcohol has the higher atomic number, and takes priority over the amine. The suffix for an alcohol ends in "ol", so that the suffix is "anol".
- The amine group is not on the carbon with the OH (the #1 carbon), but one carbon over (the #2 carbon); therefore we indicate its presence with the prefix "2-amino".
- Putting together the prefix, the root and the suffix, we get "2-aminoethanol".
There is also an older naming system for organic compounds known as common nomenclature , which is often used for simple, well-known compounds, and also for complex compounds whose IUPAC names are too complex for everyday use.
Hydrocarbon
In chemistry, a hydrocarbon is a group of chemical compounds consisting only of carbon (C) and hydrogen (H). They all consist of a carbon backbone and atoms of hydrogen attached to that backbone. (Often the term is used as a shortened form of the term aliphatic hydrocarbon.)
For example, methane (swamp gas/marsh gas) is a hydrocarbon with one carbon atom and four
hydrogen atoms: CH4. Ethane is a hydrocarbon (more specifically, an alkane) consisting of two carbon atoms held together with a single bond, each with three hydrogen atoms bonded: C2H6. Propane has three C atoms (C3H8) and so on (CnH2·n+2).
There are basically three types of hydrocarbons:
- aromatic hydrocarbons, which have at least one aromatic ring in addition to whatever bonds they have
- saturated hydrocarbons, also known as alkanes, which don't have double, triple or aromatic bonds
- unsaturated hydrocarbons, which have one or more double or triple bonds between carbon atoms, are divided into:
· alkenes
· alkynes
· dienes
The number of hydrogen atoms in hydrocarbons can be determined, if the number of carbon atoms is known, by using these following equations:
- Alkanes: CnH2n+2
- Alkenes: CnH2n (assuming only one double bond)
- Alkenes: CnH2n-2 (assuming only one triple bond)
Liquid geologically-extracted hydrocarbons are referred to as petroleum (literally "rock oil") or mineral oil, while gaseous geologic hydrocarbons are referred to as natural gas. All are significant sources of fuel and raw materials as a feedstock for the production of organic chemicals and are commonly found in the subsurface using the tools of petroleum geology.
Hydrocarbons are of prime economic importance because they encompass the constituents of the major fossil fuels (coal,petroleum, natural gas, etc.) and biofuels, as well as plastics, waxes, solvents and oils. In urban pollution, these components--along with NOx and sunlight--all contribute to the formation of tropospheri ozone.
Alkane
An alkane in organic chemi
stry is a type of hydrocarbon in which the molecule has the maximum possible number of hydrogen atoms and so has no double bonds (they are saturated).
The general formula for acyclic/linear alkanes, also known as aliphatic hydrocarbons is CnH2n+2; the simplest possible alkane is methane (CH4). The next in the series is ethane (C2H6) and the series continues with larger and larger molecules. Each C atom is hybridized sp3. The series of alkanes is often
Properties
Arrangements
The atoms in alkanes with more than three carbon atoms can be arranged in multiple ways, forming different isomers. "Normal" alkanes have the most linear, unbranched configuration, and are denoted with an n. The number of isomers increases rapidly with the number of carbon atoms; for acyclic alkanes with n = 1..12 carbon atoms, the number of isomers equals 1, 1, 1, 2, 3, 5, 9, 18, 35, 75, 159, 355 .
The names
of all alkanes end with -ane. The alkanes, and their derivatives, with four or fewer carbons have non-systematic common names, established by long precedence. For a more complete list, see List of alkanes.
| methane | CH4 |
| ethane | C2H6 |
| propane | C3H8 |
| n-butane | C4H10 |
| n-pentane | C5H12 |
| n-hexane | C6H14 |
| n-heptane | C7H16 |
| n-octane | C8H18 |
Branched alkanes have some non-systematic (or "trivial") names in common use, but there is also a systematic way of naming most such compounds, which starts from identifying the longest non-branched parent alkane in the molecule, counting up from one sequentially starting from the carbon involved in the most prominent functional group (or, more formally, attached to the collection of
heteroatoms with highest priority according to some rules), and then numbering the side chains according to this sequence.
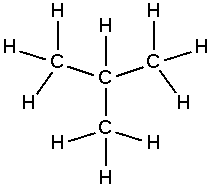
Naming Alkanes
Alkanes are named according to IUPAC nomenclature. The suffix of an alkanes name is always -ane. The prefix depends on the number of carbon atoms in the molecule and on any branched chains that may be attached. Refer to IUPAC nomenclature for greater detail.
Reactions
Cracking reactions
"Cracking" breaks larger molecules into smaller ones. This can be done with a thermic or catalytic method. The thermal cracking process follows a homolytic mechanism, that is, bonds break symmetrically and thus pairs of free radicals are formed. The catalytic cracking process involves the presence of acid catalysts (usually solid acids such as silica-alumina and zeolites) which promote a heterolytic (asymmetric) breakage of bonds yielding pairs of ions of opposite charges, usually a carbocation and the very unstable hydride anion. Carbon-localized free radicals and cations are both highly unstable and undergo processes of chain rearrangement, C-C scission in position beta (i.e., cracking) and intra-and intermolecular hydrogen transfer or hydride transfer . In both types of processes, the corresponding reactive intermediates (radicals, ions) are permanently regenerated, and thus they proceed by a self-propagating chain mechanism. The chain of reactions is eventually terminated by radical or ion recombination.
Here is an example of cracking with butane CH3-CH2-CH2-CH3
- 1st possibility (48%): breaking is done on the CH3-CH2 bond.
CH3* / *CH2-CH2-CH3
after a certain number of steps, we will obtain an alkane and an alkene: CH4 + CH2=CH-CH3
- 2nd possibility (38%): breaking is done on the CH2-CH2 bond.
CH3-CH2* / *CH2-CH3
after a certain number of steps, we will obtain an alkane and an alkene from different types: CH3-CH3 + CH2=CH2
- 3rd possibility (14%): breaking of a C-H bond
after a certain number of steps, we will obtain an alkene and hydrogen gas: CH2=CH-CH2-CH3 + H2
Halogenation reaction
R + X2 → RX + HX
These are the steps when methane is chlorinated. This a highly exothermic reaction that can lead to an explosion.
1. Initiation step: splitting of a chlorine molecule to form two chlorine atoms. A chlorine atom has an unpaired electron and acts as a free radical.
Cl2 → Cl* / *Cl
energy provided by UV.
2. Propagation (two steps): a hydrogen atom is pulled off from methane then the methyl pulls a Cl from Cl2
CH4 + Cl* → CH3* + HCl
CH3* + Cl2 → CH3Cl + Cl*
This results in the desired product plus another Chlorine radical. This radical will then go on to take part in another propagation reaction causing a chain reaction. If there is an excess of Chlorine, other products like CH2Cl2 may be formed.
3. Termination step: recombination of two free radicals
- Cl* + Cl* → Cl2, or
- CH3* + Cl* → CH3Cl, or
- CH3* + CH3* → C2H6.
The last possibilty in the termination step will result in an impurity in the final mixture; notably this results in an organic molecule with a longer carbon chain than the reactants.
Combustion
R + O2 → CO2 + H2O + H2
Is a very exothermic reaction. If the quantity of O2 is insufficient, it will form a poison called carbon monoxide (CO). Here is an example with methane:
CH4 + 2 O2 → CO2 + 2 H2O
with less O2:
2 CH4 + 3 O2 → 2 CO + 4 H2O
with even less O2:
CH4 + O2 → C + 2 H2O
Cycloalkane
Cycloalkanes are chemical compounds with a single ring of carbons to which hydrogens are attached according to the formula CnH2n. They are named analogously to their normal alkane counterpart of the same carbon count: cyclopropane, cyclobutane, cyclopentane, cyclohexane, etc.
Cycloalkanes are classified into small, normal and bigger cycloalkanes, where cyclopropane and cyclobutane are the small ones, cyclopentane, cyclohexane, cycloheptane are the normal ones, and the rest are the bigger ones.
Nomenclature
he naming of polycyclic alkanes is more complex, with the base name indicating the number of carbons in the ring system, a prefix indicating the number of rings (eg, "bicyclo"), and a numeric prefix before that indicating the number of carbons in each part of each ring, exclusive of vertices. For instance, a bicyclooctane which consists of a six-member ring and a four member ring, which share two adjacent carbon atoms which form a shared edge, is [4.2.0]-bicyclooctane. That part of the six-member ring, exclusive of the shared edge has 4 carbons. That part of the four-member ring, exclusive of the shared edge, has 2 carbons. The edge itself, exclusive of the two vertices that define it, has 0 carbons.
Reactions
The normal and the bigger cycloalkanes are very stable, like alkanes, and their reactions (cf. radicalic chain reactions ) are like alkanes.
The small cycloalkanes - particularly cyclopropane - have a lower stability due to the Baeyer-tension . They react similar to alkenes, though they don't react with the EA (cf. electrophilic addition), but with the SN2 (cf. nucleophilic substitution) reaction mechanism. These reactions are ring opening reactions or cleavage reactions of alkyl cycloalkanes.
Alkene
An alkene is one of the three classes of unsaturated hydrocarbons that contain at least one carbon-carbon double bond and have the general molecular formula of CnH2n (the other two being alkynes and arenes).
The simplest alkene is C2H4, which has the common name "ethylene" and theIUPAC name "ethene".
Structure of Alkenes
Shape of Alkenes
As predicted by the VSEPR model of electron pair replusion (see covalent bond), the bond angles about each carbon in a double bond are about 120°, although the angle may be larger because of strain introduced by nonbonded interactions created by groups attached to the carbons of the double bond. For example, the C-C-C bond angle in propene (propylene) is 123.9°.
Molecular Geometry Carbon-Carbon Double Bond
Like single covalent bonds, double bonds can be described in terms of overlapping atomic orbitals, except that unlike a single bond (which consist of a single sigma bond), a carbon-carbon double bond consists of one sigma bond and one pi bond.
Each carbon of the double bond uses its three sp2 hybrid orbitals to form sigma bonds to three atoms. The unhybridized 2p atomic orbitals, which lie perpendicular to the plane created by the axes of the three sp2 hybrid orbitals, combine to form the pi bond.
Because it requires a large amount of energy to break a pi bond (264 kJ/mol in ethylene), rotation about the carbon-carbon double bond is very difficult and therefore severely restricted.
Reactions
Synthesis
- The most common industrial synthesis path for alkenes is cracking of petroleum.
- Alkenes can be synthesized from alcohols via an elimination reaction that removes one water molecule:
H3C-CH2-OH + H2SO4 → H3C-CH2-O-SO3H + H2O → H2C=CH2 + H2SO4 - Catalytic synthesis of higher α-alkenes can be achieved by a reaction of ethene with triethylaluminium, an organometallic compound in the presence of nickel, cobalt or platinum.
Addition reactions
Catalytic addition of hydrogen
Catalytic hydrogenation of alkenes produce the corresponding alkanes. The reaction is carried out under pressure in the presence of a metallic catalyst. Common industrial catalysts are based on platinum, nickelor palladium, for laboratory syntheses, Raney's nickel is often employed. This is an alloy of nickel and aluminium.
This is the catalytic hydrogenation of ethylene to yield ethane:
CH2=CH2 + H2 → CH3-CH3
Electrophilic addition
Most addition reactions to alkenes follow the mechanism of electrophilic addition.
- Halogenation: Addition of elementary bromine or chlorine to alkenes yield vicinal Dibromo- and dichloroalkenes, respectively. The decoloration of a solution of bromine in water is an analytical test for the presence of alkenes:
CH2=CH2 + Br2 → BrCH2-CH2Br - Hydrohalogenation: Addition of hydrohalic acids like HCl or HBr to alkenes yield the corresponding haloalkanes.
CH3-CH=CH2 + HBr → CH3-CHBr-CH3
If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with less hydrogen substituents (Markovnikov's rule). - Addition of a carbene or carbenoid yields the corresponding cyclopropane
Oxidation
- In the presence of oxygen, alkenes burn with a bright flame to carbon dioxide and water.
- Catalytic oxidation with oxygen or the reaction with percarboxylic acids yields epoxides
- Reaction with ozone leads to the breaking of the double bond, yielding two aldehydes or ketones
R1-CH=CH-R2 + O3 → R1-CHO + R2-CHO + H2O
This reaction can be used to determine the position of a double bond in an unknown alkene.
Polymerisation
Polymerization of alkenes is an economically important reaction which yields polymers of high industrial value, such as the plastics polyethylene and polypropylene. Polymerization can either proceed via a free-radical or an ionic mechanism. For detail regarding the reaction mechanisms, see the polymerization article.
Nomenclature of Alkenes
IUPAC Names
To form the root of the IUPAC names for alkenes, simply change the -an- infix of the parent to -en-. For example, CH3-CH3 is the alkane ethANe. The name of CH2=CH2 is therefore ethENe.
In higher alkenes, where isomers exist that differ in location of the double bond, the following numbering system is used:
- Number the longest carbon chain the contains the double bond in the direction that gives the carbon atoms of the double bond the lowest possible numbers.
- Indicate the location of the double bond by the location of its first carbon
- Name branched or substituted alkenes in a manner similar to alkanes.
- Number the carbon atoms, locate and name substituent groups, locate the double bond, and name the main chain
| CH3CH2CH2CH2CH==CH26 5 4 3 2 1 1-Hexene | CH3 |CH3CH2CH2CH2CH==CH26 5 4 3 2 1 4-Methyl-1-hexene | CH3 | CH3CH2CH2CH2CH==CH26 5 4 3 |2 1 CH2CH3 2-Ethyl-4-methyl-1-hexene |
Common Names
Despite the precision and universal acceptance of the IUPAC naming system, some alkenes are known almost exclusively by their common names:
| | CH2="CH2" | CH3CH="CH2" | CH3C(CH3)="CH2" |
| IUPAC name: | Ethene | Propene | 2-Methylpropene |
| Common name: | Ethylene | Propylene | Isobutylene |
